We have been using different types of commercially available membranes in he lab I’m working in to test their desalination properties. One of the membrane types is not a commercially available one though, it is from the Sandia lab in New Mexico and they sent it to us to test along side the others. They had a special request however. They wanted us to test the membranes against brackish water created by fracking.
They sent us a sample of the fracking water and it was analyzed by a different lab in the University. Once the components were found we made a synthetic version to use for the experiment. What you see in the picture above is the different types of salts we needed to create the water sample. What’s missing is an extra 231 grams of Sodium Chloride that didn’t fit on the tray, for a total of over 300 grams of salt.
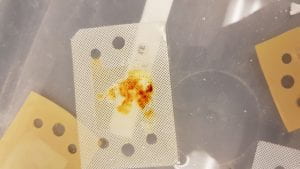
After running the experiment for 6 hours we noticed large amounts of salt stuck to the membranes. We had never seen this before with the other water types we test, and the highest we’ve used before is 100 grams of salt per liter of water. There’s even a brownish coloration the salt caused on some of the membranes that if you didn’t know any better you might think it was blood.
Needless to say that the amount of salt we found was disturbing, and the membranes weren’t even able to clean over 5% of the salt content in the water after 6 hours of continuous cleaning.