Hello!
Since this week was a much shorter week, we did not have the opportunity to do as much in the lab as what we would have been able to on a normal week. Despite this, many strides were made in reaching the goal of my project. After meeting with my lab host, Dr. Verduzco, on Wednesday, we have decided that testing other binders may not be the most logical step to take for my project. He advised me that adding this unknown element to my project may or may not help, and it could easily create more issues than what it could potentially solve. Therefore, I will not be testing with PVdF or any other additional binders. My focus will be to optimize my current slurry compositions to yield the best electrodes, and study their performance in comparison to the other types of electrodes we have tested (electrodes with no ion exchange polymers, electrodes flow coated with ion exchange polymers, and electrodes with no ion exchange polymers that are combined with commercial ion exchange membranes). A key factor to my project that Dr. Verduzco brought up was the fact that I was using QPVA without adding back in PVA to my anion exchange composite electrodes. When we synthesize QPVA from PVA, we reduce the number of functional groups by a quarter, meaning that there isn’t enough for the electrodes to reach their full potential. By adding back in PVA we can ensure there are enough functional groups in our slurry. This could be a reason why the electrodes were responding so oddly to water. I put this suggestion into action upon returning to the lab, and made electrodes coated with the new QPVA + PVA composite slurry. What we discovered was that the defacing issue was back! This wasn’t too surprising, since we had added a new material to the slurry but had kept our synthesis process the same. The activated carbon slurry coating was not coming off at an alarming rate, so it was still usable for CDI testing. We did, however, adjust the synthesis process by adding HCl to the QPVA + PVA slurry. There was a clear improvement in the electrodes once this change was made, so we will continue to make the slurries with the added HCl component. As to why this would positively impact the synthesis process I’m not entirely sure, but I will continue to research this and hopefully find an explanation.
We tested a few of my electrodes in the CDI cell, so I have been able to collect some data to report on. This set of data comes from a test we ran on one of the first electrodes I made, so the information may be a bit outdated at this point. However, I think having this starting point is good to get an idea of what I’m working with and can improve in the future.
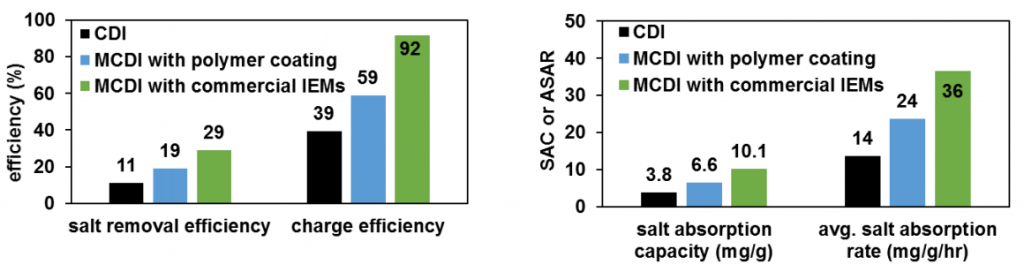
Here are the graphs of my mentor’s experiments. I am using these graphs to compare my electrode performance to each of the other types we have been testing.
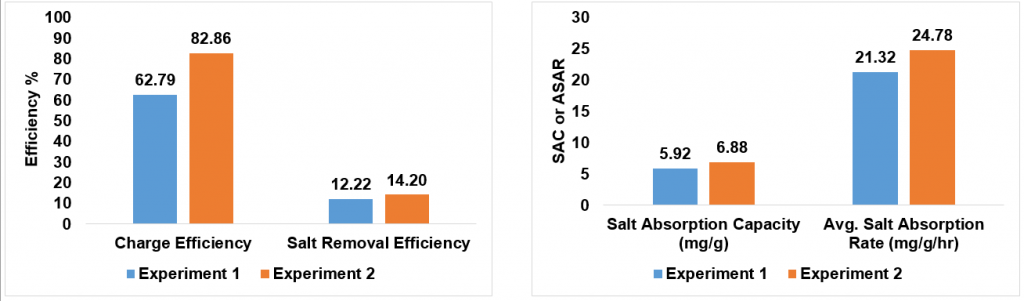
Here are the graphs for the first two experiments we ran in the CDI cell with my composite electrodes. Experiment 2 was much more promising than Experiment 1, showing that all four parameters were close to the results of the polymer coated electrodes. It can only get better from here (hopefully)!
We were also able to run a few of my better electrodes in the CDI cell. I haven’t collected all the information for my calculations yet, but I should have everything by the end of Monday or Tuesday. Hopefully the 4 main parameters will reflect an improvement! For next week, I plan to run at least 3 more tests in the CDI cell with various electrode set ups. These tests will be used to see if the addition of PVA in my QPVA slurry was beneficial to the electrode performance. We will also be taking a few samples to the ICP, a chemical analysis machine, to see whether my samples had any ion selectivity. Specifically, we will be looking at the concentrations of Calcium in the samples (from a mixed salt solution of CaCl and NaCl). I look forward to running these tests and seeing the results!
-Cierra
I enjoy your vocabulary of “slurries.” I think that’s funny. Speaking of CaCl. I needed some of that to make my test water, but we only had CaCl2.2H2O. It was kind of clumpy but soft in the container. So to get pure CaCl2, we had to throw some dihydrate into the oven at 105 Celsius and let it cook for 2 hours. It came out in hard clumps, had to break them to get a powder again. I thought it was cool. I hope you enjoy running your tests and analyzing the data though!
That is cool! And yes, I love using “slurry/slurries” for descriptions because it’s just so much fun to say. Thanks for reading my blog! 🙂
Great job! Thank you for including the graphs with your results!