Hello!
In the past week, we have been able to conduct most of the analysis portion of my project so far. All the calculations for our testing so far is finished, so we can now take a step back and begin to decipher what it all means. Below I have included graphs of all the experiments conducted so far, and the significance of their results.
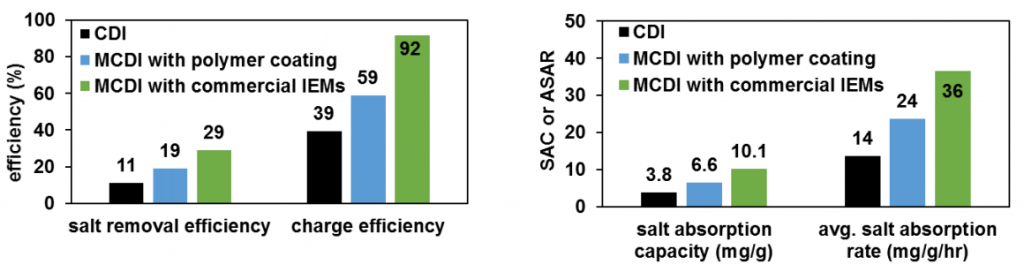
Again, here we have my mentor’s graphs to compare my results to.
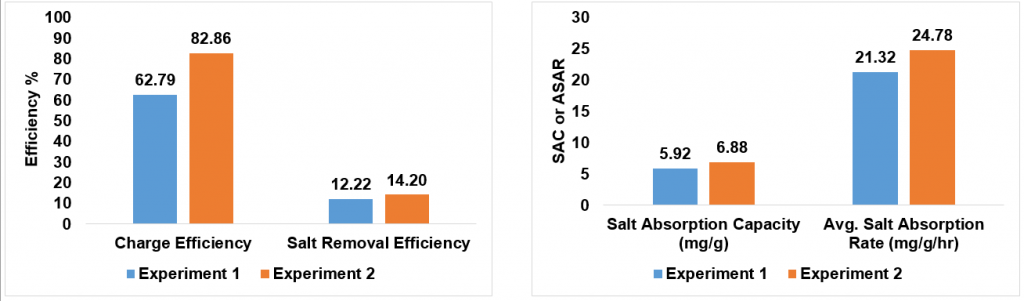
Here are the graphs for the first two experiments we ran in the CDI cell with my composite electrodes. Experiment 2 was much more promising than Experiment 1, showing that all four parameters were close to the results of the polymer coated electrodes. This was most likely due to the addition of a commercial ion exchange membrane, so we will continue trying to work without it to achieve the same results.
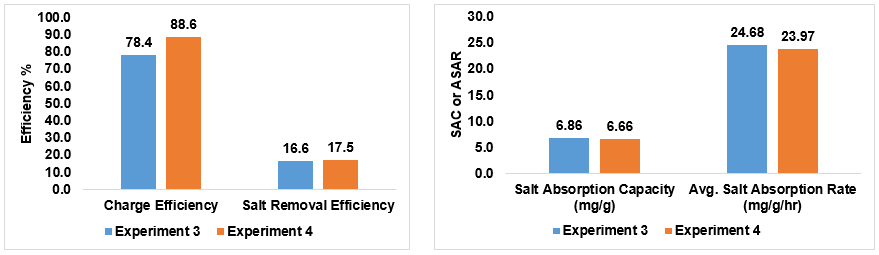
For Experiment 3 and 4, we added glutaraldehyde as a cross-linking agent in hopes of increasing performance and lowering the likelihood of the electrodes responding negatively when introduced to water. This seemed to have significantly improve performance in every parameter, however these were also tested with commercial ion exchange membranes. Experiment 4 was more successful due to the introduction of PVA to the anion exchange membrane, which increased the number of functional groups present in the slurry.
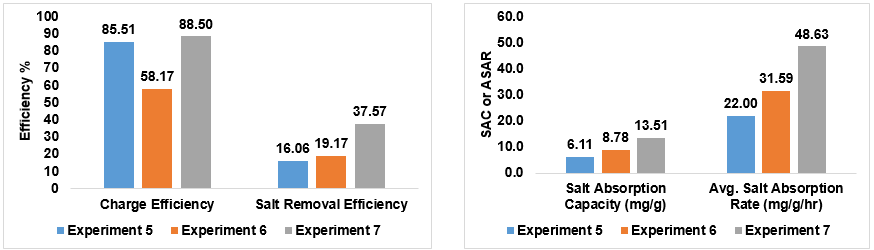
In these experiments, we removed the commercial ion exchange membrane to see if we could achieve results that were just as good. Experiment 5 had an exceptional charge efficiency in the absence of these commercial ion exchange membranes. Although the charge efficiency of Experiment 6 dropped, all the other parameters reached levels much closer to the performance of the MCDI with Commercial IEMs from the first set of graphs. With such positive results, my mentor and I were curious to see what the addition of commercial IEMs would do, so we tested retested Experiment 6 with the addition to create Experiment 7. Compared to the MCDI with commercial IEMs results, all but the charge efficiency increased beyond their numbers! This was incredibly amazing to see after calculating everything.
After analyzing these calculations, we determined the two best set ups were Experiments 5 and 6, so we chose those two to run our ion selectivity tests on. We were able to gather samples for the ICP-OES and the FTIR for both experimental set ups, and will begin analyzing them next week. A few things we know so far from these tests: even with the addition of PVA, the number of functional groups was so low that they hardly even registered on the FTIR; both experiments displayed slight sodium selectivity (as noted from the ICP-OES testing), but not enough to say we achieved something significant. This means we could increase the molar ratio from 2:1 QPVA to PVA to something closer to 1:1 QPVA to PVA to add back the functional groups that will improve the electrodes. Another thing I discussed with my mentor was the possibility of adding a coat of the ion exchange polymers in addition to the composite slurry, since we had such satisfactory results in Experiment 7. Whether I will be able to do this before the end of my internship is unknown, but hopefully I will be able to try this at least once! We think it would give the electrodes a better chance at achieving significant ion selectivity with sodium, so hopefully we can test this theory out!
Since this is my last week in the lab, I plan to focus on testing Experiments 5 and 6 again to ensure the validity of our first tests. I also plan on attempting a composite slurry electrode coated with ion exchange polymers to test out our theory on this set up. I look forward to updating you all on what we find!
-Cierra