Category Archives: NEWT YS 2017
Week Review 7/10
Updated Problem Statement
Week Review 7/5
Week Review 6/26
Hello!
My project shifted focus slightly since last week. I will now be working on improving electrode synthesis through making a new type of activated carbon slurry. For this slurry, we will be combining the activated carbon with the ion exchange membrane polymers to both simplify the synthesis process and create more uniform electrodes. This way we won’t have as many opportunities for variation between the electrodes since it will only require one coating. Here are the major steps that I will need to take to carry out this project.
Experiment Design: I have created a list of various activated carbon slurry compositions to use for making the electrodes. I am using an excel spreadsheet to calculate the amounts of each chemical needed for our slurries based on what concentrations we want to test. I typically keep the activated carbon powder at 85-90 wt. % and the ion exchange polymer at 10-15 wt. % in all the compositions. Most of the variation comes from what binders we are using for the ion exchange polymers and their specific concentrations. As I am researching, I will be looking for different binders to test. One that I plan on testing is polyvinylidene difluoride, or PVDF. It is mentioned in several papers I have read about MCDI technology, so I think I will give it a try!
Synthesize: A majority of the project will be spent making and testing these electrodes since I want to focus on trying as many options possible in the time I am given.
READ: It is essential that I continue to read as many scientific journals that relate to my project as possible. By seeing what others have tried before, I can look for new “ingredients” to incorporate into my slurry compositions.
Determining success: Once we have tested the electrodes we will be able to compare their performance to the original double coated electrodes, and from there I will be able to report the conclusive results of my project on all presentation platforms I am using. Also, I will be creating a list of quantitative and qualitative benchmarks to determine the success of each electrode I create. This way I can compare each electrode’s performance in an organized and straightforward manner, and account for all aspects of the performance.
In the past week, I have made lots of progress in the Experiment Design, Synthesize, and Read sections of my plan, and will continue to work on those moving forward. Of course, as we all know too well, progress is joined by its friend complication! I hit very many walls during this past week, but they have all helped in refining what my project has grown into. The main issue I face is in the way my electrodes are interacting with water. Throughout the entire synthesis process, the electrodes coated with my composite slurry look great. They have a smooth, uniform surface, and aren’t very brittle when cutting them to fit the 10 cm by 1 cm CDI cell size. However, when I submerge the electrode into water for the first time a strange interaction occurs. The surface of my electrodes seem to be splintering off into the water, as if the water is stripping the electrode of the dried and cross-linked surface. I have seen this occur most severely in anion exchange slurry compositions, but I have seen it occur in the cation exchange slurries as well. My mentor has suggested that we may be over or under cross-linking the electrodes, or that the pH of my slurries is somehow causing this. We aren’t entirely sure what the root of the issue is, but I will continue experimenting with different slurry compositions to see if there are any solutions. I am approaching this issue with a trial and error mindset, and will try to identify any trends in this behavior as it relates to the compositions I am using.
The top of the electrode has been stripped of the carbon slurry coating, and the grey color of the graphite sheet is showing through!
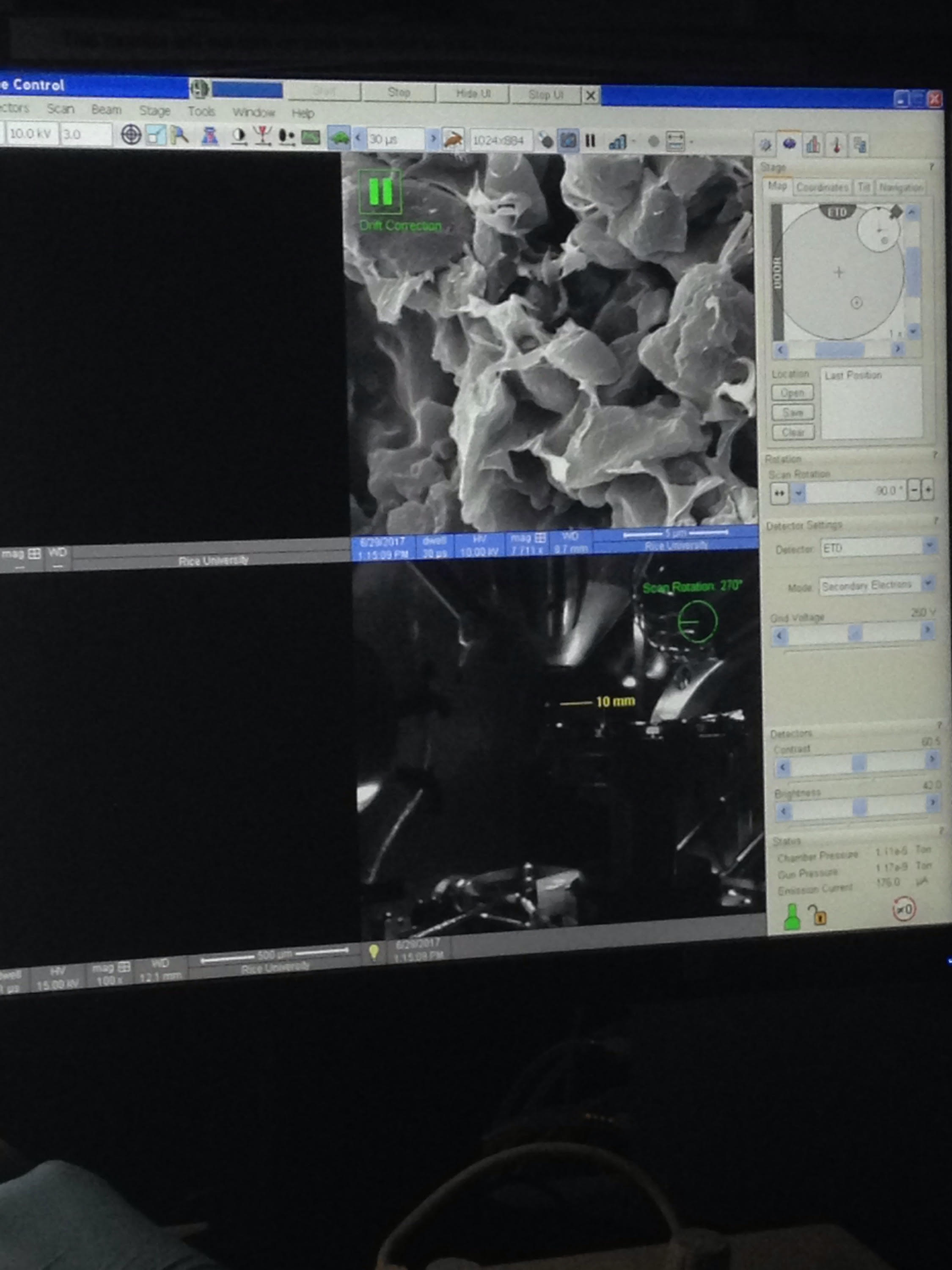
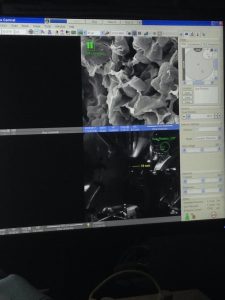
In addition to all I have talked about, this week I was able to see a scanning electron microscope for the first time! Seeing the images were absolutely incredible. I love microscopes because they make me feel as if I’m looking into an entirely different world. We used the SEM to see the true thickness of our electrodes, and what to document what their surfaces look like. It was just as amazing as I imagined it would be! Hopefully we will be able to take more samples to the SEM during my time here with NEWT.
Images of electrodes seen through the magic of the scanning electron microscope!
Looking forward to next week, I will be continuing my search for new materials to use in my slurries. I would like to find at least two new binders to test for my project by the end of next week. Since it is a shorter week, I won’t have as much time to create new slurries and test them in the CDI cell. I will aim for making 3 new slurry compositions and running tests for 3 different electrode sets. I will post an update next week with how everything turns out. Happy Fourth of July!!
-Cierra
NEWT Update and Problem Statement Rough Draft
Back in Houston
Internship Experience Update
Orientation at Rice
Welcome to the 2017 NEWT REU!
Welcome to NEWT’s 2017 REU! Here, our interns at ASU, Rice, and UTEP will document their research and share their experiences.
We wish to acknowledge the support of NSF, the postdocs and students who mentor our interns, their faculty hosts, Rice’s R-STEM office, Shahnawaz Sinha, Adam Carberry and Regina Sanborn at ASU, Roberto Fierro at UTEP, and NEWT for making this experience possible.