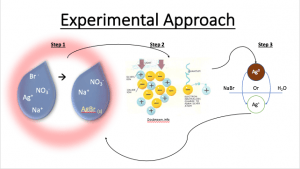
Bromide ion (Br-) in drinking waters can react with disinfectants (chlorine, ozone, etc) to produce inorganic (bromate) and organic (e.g., bromoform) disinfection by-products (DBPs) of human health concern. The national average bromide concentration is ~ 100 ug/L in drinking waters, but source waters can contain >250 ug/L annually or experience seasonal variations resulting in these higher bromide levels. Recent work has demonstrated that silver ion forms insoluble solids of bromide or chloride (AgBr, AgCl) and is capable of removing these halide ions from water (Gan et al., 2018; Kidd et al., inpress). Due to the cost of material, there is a need to develop a process to recycle silver. In this context, this work illustrates how silver ions can be re-used to remove bromide ion from drinking water by recycling the same silver nanoparticles repeatedly. First, 1.69 g of sodium chloride and 3.10g of sodium bromide were separately dissolved in ultra-pure water. Then, 1.75 g silver nitrate was added to both solutions to form silver chloride and silver bromide respectively. Confirmation of the silver chloride/silver formation is obtained by the appearance white and yellow precipitate respectively. The crystals were washed with ultra-pure water and vacuumed dried. Next, the silver bromide crystals were added to ultra-pure water to make sure there are no other ions in the solution. This solution was irradiated with UV light, during which photons of light initiate redox processes of silver bromide to form silver nanoparticles and chloride/bromide ions. During this process, water serves as the green-nontoxic electron donor. Confirmation of the reduction of silver is obtained by the formation of a brown precipitate. The remaining bromide ion is removed before recycling the silver nanoparticles. Finally, the reduced silver nanoparticles are placed back into water containing bromide ion. The color change from brown to yellow/white illustrates full cycle regeneration.
Good diagram, Carlos!